INTRODUCTION: Chronic myelomonocytic leukemia (CMML) is a clonal hematologic disorder characterized by clinical and prognostic heterogeneity. Hypomethylating agents (HMA), either alone or in combination, are common therapeutic drugs used to treat CMML. Here we characterize 165 patients (pts) with CMML and describe their outcomes after being treated with frontline HMA-based therapy
METHODS: We retrospectively reviewed the medical records of pts with CMML treated with frontline HMA-based therapy at the University of Texas MD Anderson Cancer Center (MDACC) from 2013 to 2020. We characterized the pts according to the French-American-British (FAB) classification and the CMML-specific prognostic system (CPSS). Response to therapy was defined by IWG response criteria. Overall Survival (OS) was measured from the time of starting therapy until death or until the last follow up. Survival was not censored by transplant. Kaplan-Meir method was used to evaluate survival. For the statistical analysis, IBM SPSS statistic software version 26 was used.
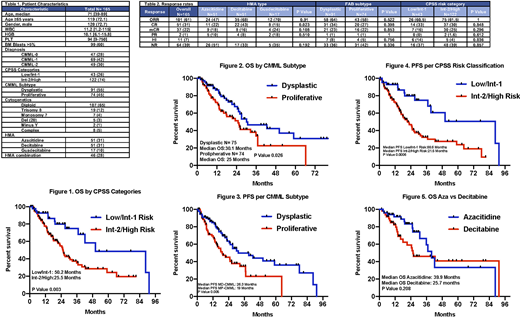
RESULTS: A total of 165 pts were identified. Pt characteristics are shown in Table 1. Median age was 71 years [range: 39-89]. One-hundred twenty pts were male (73%). Most of the patients had myelodysplastic (MD) CMML subtype (55%), and 122 pts (74%) were in the Int-2/High risk CPSS category. Most of the pts were treated with HMA single agent (Aza N= 51, decitabine N= 51, and guadecitabine N=17, total N=119). The median number of cycles of therapy was 6 (range 1-74). Overall response rate (ORR) for the whole cohort was 61%, and 51 pts (31%) achieved a best response of complete remission (CR). The median number of cycles to best response was 3 for both, Aza and decitabine. Within pts treated with HMA single agent (SA) the ORR was 60%, and CR rate of 34% vs 65% and 22% for pts treated with HMA combination therapy. Among pts treated with HMA SA (Table 2) the ORR was 47% for Aza, 68% for decitabine, and 70% for guadecitabine. The CR rate for decitabine was 43%, versus 22% for azacitidine. Patients in the low/int-1 CPSS risk categories had a combined ORR of 60.5% vs 61.5% for pts in the int-2/high risk categories (p=0.999). The CR rate for low/int-1 group was 33% and 30% for the int-2/high risk group (p=0.848). ORR were among pts with MD-CMML was 64% vs 58% in pts with MP-CMML (p=0.522) with CR rates of 34% and 27% (p=0.398), respectively. The median OS for the whole population was 30.1 months. Patients in the low/int-1 risk group had a median OS of 50.2 months vs 25.5 months for the pts in the int-2/high risk group (Figure 1) (p=0.003). The OS of pts with MD-CMML was 30.1 months vs 25 months for pts with MP-CMML (Figure 2) (p=0.026). The progression free survival for the whole group was 26.3 months. The PFS for MD-CMML pts was 31.4 months and for the MP-CMML group was 19 months (p= 0.005) (Figure 3), for the low/int-1 risk pts was 88.6 months, and for the int-2/high risk pts was 21.5 (p=0.0006) (Figure 4). The median OS for pts treated with azacitidine was 39.9 months, and for pts treated with decitabine was 25.7 months (p=0.208) (Figure 5). Patients with MD-CMML treated with azacitidine had a median OS of 30.8 months vs 42.6 months for pts treated with decitabine (p=0.345). The median OS for pts with MP-CML treated with Aza was 28.4 months vs 18 months on pts treated with decitabine (p=0.358).
CONCLUSION: The HMAs are effective in treating CMML regardless of FAB subtype or CPSS risk group. There is no statistically significant difference in CR or OS based on HMA agent. No difference in OS was observed between HMA SA versus HMA in combination, even in the int-2/high risk group.
Sasaki:Otsuka: Honoraria; Pfizer Japan: Consultancy; Novartis: Consultancy, Research Funding; Daiichi Sankyo: Consultancy. Kadia:Abbvie: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Astra Zeneca: Research Funding; Novartis: Honoraria; Pfizer: Honoraria, Research Funding; Ascentage: Research Funding; JAZZ: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Cyclacel: Research Funding; Pulmotec: Research Funding; Celgene: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Incyte: Research Funding; Cellenkos: Research Funding. DiNardo:Notable Labs: Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Jazz: Honoraria; Novartis: Consultancy; Syros: Honoraria; Takeda: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; MedImmune: Honoraria. Konopleva:Ascentage: Research Funding; Ablynx: Research Funding; Agios: Research Funding; Calithera: Research Funding; Eli Lilly: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Cellectis: Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Kisoji: Consultancy; Stemline Therapeutics: Consultancy, Research Funding; Rafael Pharmaceutical: Research Funding; Sanofi: Research Funding; Amgen: Consultancy; Forty-Seven: Consultancy, Research Funding; AstraZeneca: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding. Daver:Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Ravandi:Amgen: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Macrogenics: Research Funding; Xencor: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Orsenix: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding. Pemmaraju:Plexxikon: Research Funding; SagerStrong Foundation: Other: Grant Support; Pacylex Pharmaceuticals: Consultancy; Cellectis: Research Funding; Celgene: Honoraria; Roche Diagnostics: Honoraria; Blueprint Medicines: Honoraria; AbbVie: Honoraria, Research Funding; Affymetrix: Other: Grant Support, Research Funding; Samus Therapeutics: Research Funding; Novartis: Honoraria, Research Funding; Incyte Corporation: Honoraria; MustangBio: Honoraria; DAVA Oncology: Honoraria; Daiichi Sankyo: Research Funding; Stemline Therapeutics: Honoraria, Research Funding; LFB Biotechnologies: Honoraria. Short:Astellas: Research Funding; Takeda Oncology: Consultancy, Honoraria, Research Funding; Amgen: Honoraria; AstraZeneca: Consultancy. Kantarjian:Agios: Honoraria, Research Funding; Takeda: Honoraria; Pfizer: Honoraria, Research Funding; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Cyclacel: Research Funding; Astex: Research Funding; BMS: Research Funding; Ariad: Research Funding; Immunogen: Research Funding; Amgen: Honoraria, Research Funding; Novartis: Research Funding; Jazz Pharma: Research Funding; Daiichi-Sankyo: Research Funding. Garcia-Manero:Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Onconova: Research Funding; Amphivena Therapeutics: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; H3 Biomedicine: Research Funding; Jazz Pharmaceuticals: Consultancy; Acceleron Pharmaceuticals: Consultancy, Honoraria; Novartis: Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal